A Review of Antiplatelet Therapy in the Secondary Prevention of Stroke in the Elderly
D'Arcy Little, MD, CCFP
Director of Medical Education,
York Community Services, Toronto
Fan-Hsia Mang, BSc
Elective Medical Student,
University of Alberta, Edmonton
Introduction and Background
Stroke is the third leading cause of death in the industrialized world and is a major cause of long-term disability.1 It is estimated that 40% of patients who survive a first transient ischemic attack (TIA) or stroke will have a subsequent stroke within the next five years.1 As increased age is one of the major non-modifiable risk factors for stroke, secondary prevention of stroke in the elderly population is an important clinical consideration. The following article will review the antiplatelet medications available for the secondary prevention of stroke in the elderly.
According to current guidelines, patients with a first cerebrovascular event due to cardioembolism should be treated with oral anticoagulants, provided there are no contraindications. This topic has already been reviewed previously in this publication.2,3,4 Patients who experience cerebrovascular events secondary to atherothrombosis typically receive antiplatelet agents. Aspirin is the best studied antiplatelet agent used for the secondary prevention of stoke, but in the past decade, other agents have been added to the anti-stroke armamentarium, including ticlopidine (Ticlid), clopidogrel (Plavix) and a combination agent containing dipyridamole and ASA (Aggrenox). 5
Acetylsalicylic Acid (ASA)
Acetylsalicylic acid (ASA, Aspirin) has been used in clinical practice for decades. It acts by irreversibly inhibiting platelet cyclo-oxygenase, subsequently inhibiting the formation of thromboxane A2. Thromboxane A2 is a vasoconstrictor as well as an activator for platelet aggregation and release.6 [See Figure 1: Normal Platelet Function and Antiplatelet Agents]
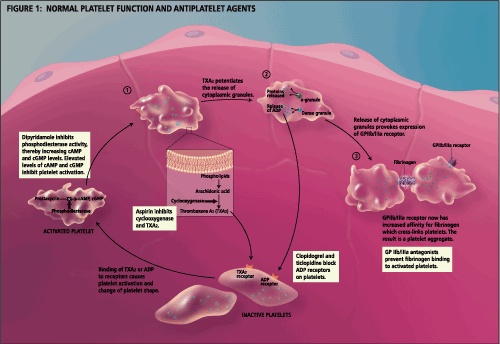
Early trials, in the 1970's and early 1980's, probing the use of ASA as an antiplatelet agent in the prevention of stroke yielded negative, equivocal or contradictory results, largely due to small sample sizes and population heterogeneity.7 In addition, early trials of alternative antiplatelet agents such as sulfinpyrazone and dipyridamole suggested no significant additional benefit over aspirin.8,9,10
In 1991, however, the United Kingdom Transient Ischemic Attack Trial (UK-TIA) and the Swedish Aspirin Low-dose Trial (SALT) suggested that ASA alone had efficacy for preventing secondary stroke in patients with cerebrovascular disease.7,11,12 The arm of the Second European Stroke Prevention Study (ESPS-2) in which patients received ASA alone demonstrated, among other things, that ASA alone has a highly significant protective effect. The relative stroke risk was decreased by 18.1% with ASA compared to placebo.13,14 In addition, a meta-analysis, The Antiplatelet Trialist's Collaboration (APTC), of 25 randomized trials of various forms of antiplatelet therapy used in the treatment of stoke, TIA, unstable angina and myocardial infarction, revealed that antiplatelet therapy was associated with a 27% reduction in nonfatal stroke (p<0.001) and a 25% reduction in stroke, myocardial infarction, or vascular death (p=0.0001). Most of these trials involved ASA.6,15
Although in the past there has been considerable debate regarding the optimal dose of ASA for secondary stroke prevention, a series of trials have indicated that there is no significant difference in the protective effect of ASA in doses ranging from 30 to 1200 mg/day. Low dose aspirin (50 to 100 mg) reduces the risk of vascular events in patients with prior stroke or TIA by 13%, with no evidence of a dose-response relationship.7,11,12,16 Furthermore, lower-dose ASA has been associated with a reduced incidence of gastric discomfort and gastric bleeding.7,17
Aggrenox (Aspirin and Dipyridamole)
Dipyridamole is a platelet inhibitor that is thought to work by inhibiting platelet phosphodiesterase, raising the anti-aggregating effects of cyclic adenosine monophosphate and cyclic guanosine monophosphate.6,13 In addition, dipyridamole may stimulate prostacyclin synthesis and potentiate the antiplatelet effect of prostacyclin (See Figure 1).6 Initial studies in the 1960's seemed to illustrate a lack of efficacy in stroke prevention compared to ASA. In addition, patients taking dipyridamole seemed to experience more adverse side effects such as headache.6 In contrast, the ESPS-2 found that both extended-release dipyridamole (200 mg twice daily) and ASA (25 mg twice daily) had an independent and statistically significant effect in reducing the risk of stroke recurrence (16% and 18% respectively), when compared to placebo (p<0.03). In addition, the combination of extended-release dipyridamole plus ASA (Aggrenox), in the above stated doses, had significant and additive effects on preventing stroke recurrence (37% relative risk reduction with p < 0.001), in comparison with placebo. The combination reduced the risk of stroke, both fatal and nonfatal, by 23% compared to aspirin alone.3,6,13
Common adverse side effects of Aggrenox include headache, dizziness, and gastrointestinal complaints such as dyspepsia, diarrhea and nausea. While the bleeding risk associated with Aggrenox is similar to that of ASA alone, the dose-limiting adverse effects of this agent are comparable to that of dipyridamole alone.6 Contraindications to Aggrenox include hypersensitivity to either ASA or dipyridamole, and a history of peptic ulcer disease.
Ticlopidine (Ticlid)
Ticlopidine, a thienopyridine agent, acts by suppressing platelet aggregation induced by adenosine diphosphate (ADP) (See Figure 1).1,13 Two large, randomized controlled trials have demonstrated that ticlopidine has a protective effect in patients with recent thromboembolic stroke.18,19 The CATS study, which compared ticlopidine (250mg twice daily) with placebo, concluded that ticlopidine reduces the relative risk of stroke, MI, or vascular death by 30 %.1,6,18 The TASS study compared ticlopidine (250 mg twice daily) with ASA (650 mg twice daily) and concluded that ticlopidine was associated with a 21% greater relative risk reduction for stroke compared with ASA (p=0.02).1,6,18
While ticlopidine boasts a fairly mild benefit over ASA in stroke prevention, it is associated with a considerable risk of serious adverse events. There is a 1% incidence of severe, reversible neutropenia, as well as a risk of skin rash, diarrhea, thrombocytopenia, and thrombotic thrombocytopenic purpura (TTP).1,6,18 Given the rather modest absolute risk reduction with ticlopidine compared to ASA (2% over three years), as well as the need for regular blood monitoring to detect neutropenia, the drug was initially reserved for patients who were intolerant of ASA, or who had recurrent strokes on ASA ("ASA failure"). These potential side effects, coupled with the development of clopidogrel, have limited the use of this medication.1,19,20
Clopidogrel (Plavix)
Clopidogrel is a new thienopyridine derivative in the same family as ticlopidine (See Figure 1). The CAPRIE (Clopidogrel vs Aspirin in patients at risk of ischemic events) trial, a large, secondary prevention study of patients with recent myocardial infarction, stroke, or peripheral vascular disease, compared the efficacy of clopidogrel (75 mg once daily) to ASA (325 mg once daily). The study showed a significant, 8.7% relative risk reduction for clopidogrel over aspirin (p<0.05) for the combined endpoints of ischemic stroke, myocardial infarction and vascular death.1,13,21 If one uses the 25% relative risk reduction of ASA found by the Antiplatelet Trialist's Collaboration, it can be extrapolated that clopidogrel imparts a 33% relative risk reduction. The stroke group sub-analysis showed a relative risk reduction of 7.3%, but the p-value was not significant (p=0.26). However, as the CAPRIE study was not designed to detect differences within patient subgroups, such analysis may be inaccurate.7,21
Clopidogrel has no significant difference in adverse side effects compared to ASA. Unlike ticlopidine, clopidogrel is not associated with neutropenia. While several cases of TTP have been reported with clopidogrel, the incidence is much smaller than that associated with ticlopidine. As a result, routine blood monitoring is not recommended for patients taking clopidogrel.13 There has been no head to head comparison of the efficacy of clopidogrel versus ticlopidine, or of clopidogel versus Aggrenox. Due to a modest superiority over ASA but a considerably higher cost, clopidogrel has not replaced ASA as first line therapy in stroke prevention.13
Future Research
Due to favourable results in studies of Aggrenox, other combination therapies may play a greater role in the future. For example, the combination of ticlopidine and aspirin has proved superior to conventional anticoagulation plus aspirin in the prevention of cardiac stent thrombosis.22 In addition, clopidogrel and ASA have been shown to act synergistically in the same context.23 The CURE trial has also recently demonstrated that clopidogrel in combination with ASA reduces the relative risk of the combined endpoints of cardiovascular death, myocardial infarction, and stroke by 20% (p=0.0005) in the context of acute coronary syndromes.24,25 Studies addressing combinations with specific relevance to stroke prevention are pending.
The platelet glycoprotein (Gp IIb/IIIa) complex is the final common pathway for platelet aggregation. Oral platelet Gp IIb/IIIa antagonists prevent the binding of fibrinogens to platelets, regardless of the trigger for platelet aggregation; therefore, these agents may have a future role in stroke prevention (See Figure 1).
Conclusion/Recommendations
In summary, the above review is synthesized in a list of current recommendations for the secondary prevention of atherothombotic stroke in the elderly:
- Provided there are no contraindications, every patient who has experienced an atherothrombotic stroke or TIA should receive an antiplatelet agent on a regular basis to reduce the risk of recurrent stroke and other vascular events.3
- The choice of antiplatelet agent must weigh the risks of stroke against the benefits, risks and costs of treatment.3
- ASA is recommended as the initial agent. The starting dose should be in the range of 50 to 325 mg. Fewer side effects are experienced at lower doses.3,13
- Some clinicians advocate for the use of aspirin plus dipyridamole as first line therapy, although this has not been universally accepted.1,3,13
- For patients intolerant to ASA, clopidogrel is recommended over ticlopidine because of a more favourable side effect profile.3,6
- For ASA failure, either clopidogrel or dipyridamole and ASA may be selected. Perhaps other combination agents may be used for this indication in the future.13
References
- Sacco RL, Elkind MS. Update on antiplatelet therapy for stroke prevention. Arch Intern Med 2000;160:1579-82.
- Little DL. Secondary prevention of stoke: the role of antiplatelet and anticoagulant agents. Geriatrics and Aging 2000;3(1):8-9.
- Albers GW, Easton JD, Sacco RL, Teal P. Antithrombotic and thrombolytic therapy for ischemic stroke. Chest 1998;114(5):683S-698S.
- Laupacis A, Albers G, Dalen J, Dunn MI, Jacobson AK, Singer DE. Antithrombotic therapy in atrial fibrillation. Chest 1998;114(5):579S-589S.
- Albers GW, Tijssen JGP. Antiplatelet therapy: new foundations for optimal treatment decisions. Neurology 1999;53(Suppl 4):S25-S31.
- Lenz TL, Hilleman DE. Aggrenox: a fixed combination of aspirin and dipyridamole. Ann of Pharmacotherapy 2000;34:1283-90.
- Forbes CD. Antiplatelet therapy for secondary stroke prevention. Scot Med J 1999;44:57-62.
- Canadian Cooperative Study Group. A randomised trial of aspirin and sulfinpyrazone in threatened stroke. N Engl J Med 1978;299:53-9.
- Bousser MG, Eschwege E, Haguenau M, Lefaucconnier JM, Thibult N, Touboul C, Touboul PJ. "AICLA" controlled trial of aspirin and dipyridamole in the secondary prevention of atherothrombotic cerebral ischemia. Stroke 1983;14:5-14.
- American Canadian Cooperative Study Group. Persantine aspirin trial in cerebral ischemia II. Endpoint results. Stroke 1985;16:406-15.
- SALT Collaborative Group. Swedish aspirin low-dose trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischemic events. Lancet 1991;338:1345-49.
- UK-TIA Study Group. United Kingdom transient ischemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psych 1991;54:1044-54.
- Davis SM, Donnan GA. Newer antiplatelet therapies in stroke prevention. Aust. Fam. Phys. 2001;30(2):129-34.
- Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J. Neurol. Sci 1996;143:1-13.
- Antiplatelet Trialist Collaboration. Collaborative overview of randomized trials of antiplatelet therapy. 1. Prevention of death, myocardial infarction and stoke by prolonged antiplatelet therapy in various categories of patients. Br Med J 1994;308:81-106.
- Tijssen JGP. Low-dose and high-dose acetylsalicylic acid, with and without dipyridamole: A review of clinical trial results. Neurology 1998;51(Suppl3):S15-6.
- Dutch TIA Trial Study Group. A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. N Engl J Med 1991;325:1261-6.
- Gent M, Blakely JA, Eason JD, et al. The Canadian American ticlopidine study (CATS) in thromboembolic stroke. Lancet 1989;1:1215-20.
- Hass Wk, Easton JD, Adams HP Jr, Pryse-Phillips W, Molony BA, Anderson S, Kamm B. A randomised trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high risk patients. N Engl J Med 1989;321:501-7.
- Bennett CL, Weinberg PD, Brosenberg-Ben-Dror K, Yarnold PR, Kwaan HC, Green D. Thrombotic thrombocytopenic purpura associated with ticlopidine: a review of 60 cases. Ann Intern Med 1998;128:541-4.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischemic events (CAPRIE). Lancet 1996;348:1329-39.
- Schomig A, Neumann FJ, Kastrati A, et al. A randomised comparison of antiplatelet and anticoagulant therapy after the placement of coronary artery stents. N Engl J Med 1996;334:1084-9.
- Moussa I, Oergen M, Roubin G, et al. Effectiveness of clopidogrel and aspirin versus ticlopidine and aspirin in preventing stent thrombosis after coronary stent implantation. Circ 1999;99:2364-6.
- Mehta SR, Yusuf S. The Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) Study Investigators. European Heart Journal 2000;(24):2033-41.
- Beck DL. Clinical Research: CURE Trial finds ASA enhanced by clopidogrel. The Chronicle of Cardiovascular and Internal Medicine 2001 April:1-8.
-