
The Role of RANK/RANKL/OPG Pathway in Bone Loss: New Insights
Bone Biology and the Role of RANK/RANKL/OPG Pathway
Speaker: Robert G. Josse, MD, Division of Endocrinology & Metabolism, St. Michael’s Hospital; Professor of Medicine, University of Toronto, Toronto, ON.
Advances in the understanding of bone biology and the role of the RANK/RANKL/OPG pathway have opened new treatment avenues for osteoporosis. To facilitate understanding of the “new biology,” Dr. Robert Josse first reviewed determinants of bone strength.
Trabecular bone, a spongy network of delicate plates of bone known as trabeculae, constitutes 20% of skeletal mass but accounts for ~80% of bone turnover. In contrast, cortical bone constitutes 80% of mass but ~20% of turnover. The interior surface of cortical bone, the endosteum, is the primary site of remodeling and metabolic activities while the exterior surface, the periosteum, is the site of new bone formation.
Remodeling, Dr. Josse noted, takes place continuously: tiny packets of bone throughout the skeleton constantly undergo this process during the life-span. Remodeling optimizes bone structure for mechanical function and repairs microdamage, adding strength where it is needed. Bone is deposited and resorbed in accordance with the stresses placed upon it. Bone is also a source of circulating growth factors, which is important in our understanding of bone biology. Finally, bone acts as a reservoir for calcium and phosphate. A feedback mechanism enables parathyroid hormone (PTH) to stimulate the release of calcium from the skeleton when needed as well as enhance bone resorption to increase the availability of calcium for vital tissues and metabolic functions.
Excessive remodeling contributes to osteoporosis as accelerated bone remodeling results in structural decay and net bone loss. Bone strength and ability to resist fracture depend on both structural integrity and mass, and both tend to deteriorate with age and with menopause in women.
Understanding bone biology involves comprehending the function of the bone cells involved in remodeling. Dr. Josse went on to review the bone remodeling sequence. The first step in remodeling is activation of osteoclasts responsible for resorption. Cells that line bone surfaces retract, allowing the osteoclasts to resorb exposed bone tissue, creating “pits.” Following the reversal stage, osteoblasts lay down new osteoid matrix, which subsequently mineralizes. Osteoclasts are derived from the monocyte macrophage series. They attach to bone, form a tight seal, and start resorbing the bone. Osteoclasts excavate trenches in bone. Osteoblasts are derived from mesenchymal precursors in the marrow responsible finally for differentiating into osteoblasts. These mesenchymal stem cells are the same cells that differentiate into myoblasts and adipoblasts. Thus those stem cells that make bone also make muscle and fat. Age-associated biological processes determine a shift of these stem cells toward fat. This is in part why in older adults there is sarcopenia and the marrow is fat-laden.
Once osteoclasts excavate an area of bone, osteoblasts fill up the bone resorption pit with unmineralised matrix. This hardens when minerals, e.g., calcium, are deposited in it. In healthy young individuals, the amount of resorption is balanced with formation. An imbalance in bone remodeling that favours resorption, such as occurs in osteoporosis, leads to decreased mineral density and microarchitectural deterioration, increasing fragility and fracture susceptibility. Although we have less trabecular versus cortical bone, the effects of an imbalance in bone remodeling will first become apparent in trabecular bone, which is more readily disrupted by excess bone resorption. In states of imbalance and weakened trabecular bone, the bone’s platelike structures convert to rod-like structures, which are mechanically weaker.
Biologists were perplexed for years, Dr. Josse explained, by how osteoclasts resorbed bone. The osteoclast does not possess receptors for the substances (cytokines, hormones, etc.) that activate resorption.
This conundrum was settled when the RANK ligand system was discovered. Receptor activator of nuclear factor-kB ligand (RANKL), a protein that binds to receptor activator of nuclear factor-kB (RANK), is the primary mediator of osteoclast differentiation, activation, and survival (Figure 1). RANK ligand is the primary mediator of bone resorption. Osteoprotegerin (OPG) provides an alternative binding site for RANKL and acts as a decoy receptor by blocking RANK ligand binding to its cellular receptor RANK. Ligand binding activates cellular signalling. Ligand that is bound to a decoy receptor cannot activate cellular signalling.1-6
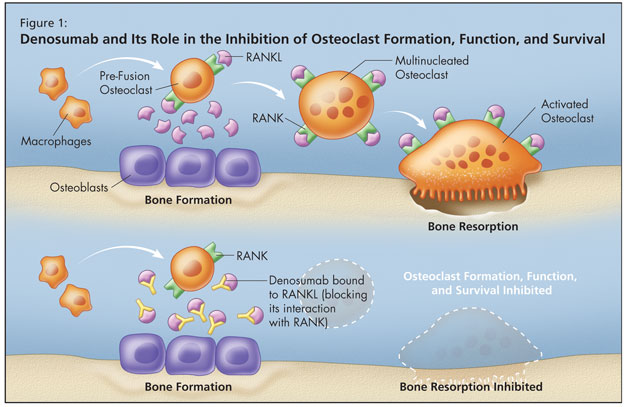
Osteoprotegerin is the natural key endogenous regulator of the RANKL–RANK pathway. When RANK ligand is bound and neutralized by OPG, osteoclasts cannot form,2,5 function,2 or survive.3 Osteoprotegerin acts as a decoy receptor by binding with RANKL, thereby inhibiting osteoclastogenesis and the survival of pre-existing osteoclasts.1,4,7,8 By binding to and neutralizing the effects of RANKL, it thereby inhibits bone resorption.1,4,7,8 More RANKL equates with more bone loss.
Denosumab is an osteoporosis treatment designed to target RANKL. It is a fully human monoclonal antibody (IgG2) that binds to RANKL with high affinity and specificity. It blocks the interaction of RANKL with RANK, mimicking osteoprotegerin.
The clinical use of OPG is impractical, Dr. Josse observed, and researchers saw the need to create other agents to neutralize RANKL, which increases bone resorption and reduces trabecular and cortical bone. It is implicated in osteoporosis and its inhibition increases bone mineral density and maintains bone architecture. Denosumab is now being investigated in treatments other than osteoporosis, including cancer-related bone destruction and bone erosion in the setting of rheumatoid arthritis.
Dr. Josse reiterated that a healthy skeleton depends on a balance between bone resorption and formation; treatments that are able to achieve RANK ligand inhibition and therefore appropriate suppression of bone resorption could offer significant clinical value to patients with bone loss.1,6
References:
-
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003;423:337–42.
-
Fuller K, Wong B, Fox S, et al. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med 1998;188:997–1001.
-
Lacey DL, Tan HL, Lu J, et al. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 2000;158:435–48.
-
Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998;93:165–76.
-
Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 1998;95:3597–602.
-
Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/ RANK system for bone and vascular diseases. JAMA 2004;292:490–5.
-
Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997;89:309–19.
-
Bekker PJ, Holloway D, Nakanishi A, et al. The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 2001;16:348–60.
Sponsored by an unrestricted educational grant from Amgen Canada Inc.