Joanna Goldberg, MSc, Associate Editor, Geriatrics & Aging.
As a woman ages and approaches her menopause, one of the most important health decisions she will be faced with is whether to take hormone replacement therapy (HRT). The age-defying effects of HRT touted in the past by both the popular press and the medical establishment ranged from sustaining supple skin, youthful libido and a cheerful demeanor, to staving off osteoporosis, heart disease and dementia. Although contradicting evidence weighing these benefits against its associated risks have been trickling in for years, the recent results of a major NIH study will now dramatically change the way women and their general practitioners view HRT.
These results, released in May from the large U.S.-federally funded trial--part of a larger group of studies from the NIH-sponsored Women's Health Initiative--definitively showed for the first time that the risks associated with HRT exceed its benefits in healthy menopausal women. In fact, these conclusions were so striking that the trial involving over 16,000 women was stopped after a follow-up period of 5.2 years--three years short of its scheduled duration.
Back in the fall of 1997, approximately half of these participants, aged 50 to 79 years and all with an intact uterus, were randomized to receive either one daily tablet of the estrogen-progestin combination pill, Prempro (0.625mg conjugated equine estrogen and 2.5mg medroxyprogesterone acetate) or a placebo. The plan was to follow the women for an average of eight years and record how many heart attacks, strokes, blood clots, hip fractures and instances of colon cancer occurred. The first hint that all was not well came late in 1999, when the independent Data and Safety Monitoring Board unexpectedly observed a small but consistent increase in the risk of blood clots and heart attacks in the cohort of women taking HRT. Since then, the monitoring board has had similar inklings that HRT may render certain risks, and it was recommended that trial participants be informed of small increases in myocardial infarction, stroke and blood clots. However, the trial continued because the balance of risks and benefits remained uncertain.
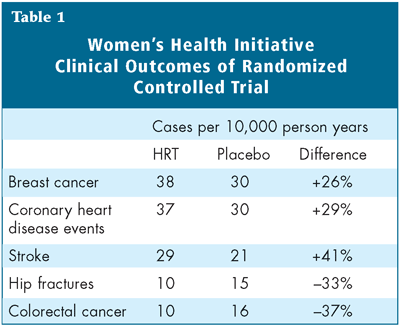
The latest results, published in the July 19 issue of The Journal of the American Medical Association, differed from these previous observations in that the risk of invasive breast cancer crossed the pre-determined boundary established as a sign of risk. The alarm bells rang clear when the data were released: there were increases in the risk of breast cancer (26% higher among HRT-users), heart disease (29% higher) and stroke (41% higher), although the risks of colon and rectal cancer (37% lower) and hip fracture (33% lower) declined among women randomized to HRT.1
To all but those who for years remained skeptical, these results came as quite a surprise, and to millions of women taking HRT for various reasons, they were quite a nasty shock. But what exactly do the numbers mean for the individual woman who has been taking HRT for years or is considering it as she reaches menopause? Although the WHI trial results were reported in terms of relative risk, in order to apply them to clinical practice they must be translated into absolute risk. The absolute risk of harm to an individual woman is actually very small: among 10,000 women taking the studied combination of HRT for one year, there will be eight more invasive breast cancers compared to a group of 10,000 women taking placebo for one year (Table 1). There will also be seven more coronary heart disease events, eight more strokes and eight more pulmonary emboli among these women compared to their placebo-taking counterparts, but six fewer colorectal cancers and five fewer hip fractures. When the total number of events is considered, the excess number of events over the 5.2 years of the trial was one in 100 for women taking HRT, a finding that demonstrates how the risk adds up over time.
So what do these numbers mean for the primary care physician, to whom thousands of postmenopausal women will turn for answers? Long-term hormone therapy has, for years, been endorsed by the medical community to prevent disease and preserve health in postmenopausal women. The WHI study shows that HRT may, in fact, be doing the opposite, even though the absolute risk is low. Taking estrogen and progestin long-term in the hope of preventing a heart attack or stroke can no longer be considered a valid medical strategy. Primary care practitioners should, therefore, stop prescribing this estrogen-progestin combination for long-term use in healthy postmenopausal women for the prevention of chronic disease.
It may, however, still be reasonable for women to continue taking HRT for the short-term relief of menopausal symptoms. A very slight, short-term risk of blood clots seems to emerge as soon as women begin taking the hormones, yet the increased risk for stroke and breast cancer didn't appear until one and four years later, respectively. Every woman facing the choice of HRT for the short-term alleviation of menopausal symptoms must decide, with the support of her doctor, whether her symptoms are so intolerable that she is willing to take that very small chance of a complication. Alternative options for managing menopausal symptoms should be considered more seriously, including relaxation exercises for mood swings, low-dose estrogen creams or rings for vaginal dryness (see article, The Recognition and Management of Atrophic Vaginitis) and many natural remedies for hot flashes.
Although the WHI results cannot be extrapolated to other formulations of HRT, or even different doses of the same combination, other studies have reported increases in the risk of breast cancer as well. One such study found the incidence of all histologic types of breast cancer combined was increased from 60-85% in recent long-term users of HRT, whether the women were taking estrogen alone or in combination with progestin.2
The definitive proof coming out of the WHI trial has actually made menopause management a lot more complex. Although women are not advised to abruptly dump their HRT regimens, each should have a serious talk with her primary care physician to balance the absolute risks against the severity of her menopausal symptoms and the benefits she reaps from HRT. Perhaps it is also time to more actively explore alternative medications and well-proven therapies for heart disease, cancer and osteoporosis, and to focus on lifestyle modifications such as exercise, low-fat diet, and reducing blood pressure and cholesterol levels. The newer class of estrogen-like drugs called SERMS, such as raloxifene, is showing promise in reducing fractures without raising the odds of breast cancer.3 The results from the WHI study encourage us to return back to the basics, while looking ahead to the future with the hopes of alternative preventive strategies.
Source
- Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321-33.
- Chen CL, Weiss NS, Newcomb P, et al. Hormone replacement therapy in relation to breast cancer. JAMA 2002;287:734-41.
- Khovidhunkit W, Shoback DM. Clinical effects of raloxifene hydrochloride in women. Ann Intern Med 1999;130:431-9.