D'Arcy Little, MD, CCFP
Director of Medical Education,
York Community Services, Toronto, ON.
Introduction
Atrial fibrillation (AF) is the most common, chronic arrhythmia seen in clinical practice,1,2 and is a common cause of morbidity, mortality and health care expenditure. The prevalence of the arrhythmia increases dramatically with age; it is estimated to have a prevalence of 5% in individuals aged 60 to 70 and of 22% in persons aged 91 to 103 years.2,3 AF commonly causes symptoms in elderly patients, including palpitations, shortness of breath, fatigue and exercise intolerance.4 In addition, the presence of AF is an independent risk factor for stroke, especially in older persons.4 The risk of stroke is increased six-fold in patients with AF, even those without coexistent rheumatic heart disease. Further, it is estimated that over one-third of all strokes in the elderly are a consequence of AF.2,4,5,6
Approach to Treatment with Electrical Cardioversion
The goals of therapy in patients with AF are to control the patient's symptoms and to reduce the risk of complications from thrombo-embolism.1 Conversion of AF back to normal sinus rhythm will accomplish the first goal immediately and the second goal, theoretically, over the long term if sinus rhythm can be maintained post conversion. These results are thought to be due to the return and maintenance of atrial mechanical function.1
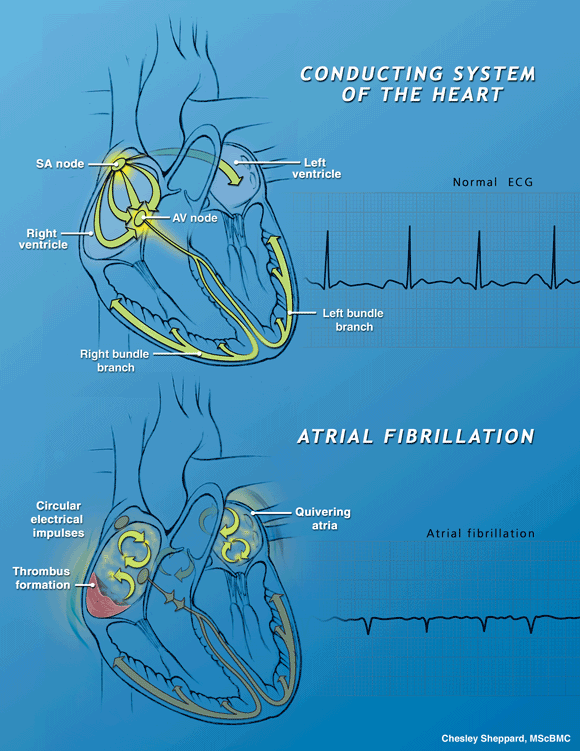
| Atrial fibrillation (AF) is characterized by the irregular and very rapid beating of the heart's atrial chambers. It results from a malfunction of the electrical conduction system of the atria, leading to chaotic electrical signals. The regular pumping action of the atria is replaced by irregular and disorganized spasms of atrial tissue, leading to reduced blood flow, blood clots (thrombi), stroke and even death. |
Considerations Prior to Electrical Cardioversion
Spontaneous Cardioversion
In up to 48% of cases of recent-onset AF, spontaneous reversion to sinus rhythm occurs. The most important factor in determining whether spontaneous reversion can occur is the duration of the AF. AF of less than 72 hours duration has a spontaneous conversion rate of approximately 40%.1
Emergent Cardioversion
Immediate, direct current (DC) cardioversion should be performed in patients who are unstable with serious signs or symptoms. This can be the case in atrial fibrillation with a very rapid ventricular rate (greater than 150 bpm) contributing to acute myocardial infarction, angina, congestive heart failure, hypotension or syncope.2
Elective Cardioversion:
Contraindications
The treatment or elimination of any reversible, predisposing conditions should be undertaken prior to elective cardioversion. Such precipitating causes include hyperthyroidism, pneumonia, acute myocardial infarction, pulmonary embolism and pericarditis. Conditions that are relatively unfavourable for elective DC cardioversion, or where elective DC cardioversion is contraindicated, should also be investigated. These conditions are listed in Table 1. However, a recent study suggested that the duration of AF may have less of an influence on the ability of AF to be cardioverted to, and maintained in normal sinus rhythm in the absence of coexisting significant heart disease.7 Appropriate initial blood work includes complete blood count, creatinine, electrolytes and thyroid function (sTSH).1 Age does not influence the success of cardioversion. Congestive heart failure, poor LV function and increased left atrial size have been found by some but not all investigators to decrease success.8
| TABLE 1 Unfavourable Conditions for Elective Cardioversion of Chronic Atrial Fibrillation |
- Duration of atrial fibrillation of more than 1 year (see text)
- Moderate to severe cardiomegaly
- Echocardiographic left atrial dimension >45 mm
- Digitalis toxicity (contraindication)
- Slow ventricular rate (contraindication)
- Sick sinus syndrome (contraindication)
- Mitral valve disease
- Congestive heart failure
- COPD
- Recurrent atrial fibrillation despite anti-arrhythmic drugs
- Inability to tolerate anti-arrhythmic drugs
|
| Modified from: Aronow WS. Management of atrial fibrillation, ventricular arrhythmias and pacemakers in older persons: Management of the older person with atrial fibrillation. JAGS 1999;47(6):740-8. |
Anticoagulation
Patients with AF have an increased risk of thrombo-embolism. Cardioversion from AF to sinus rhythm in a patient with prolonged AF who is not anticoagulated is associated with a 5-7% risk of stroke.8 While there are no randomized trials evaluating the efficacy of anticoagulation with warfarin, several large studies suggest that, with prior anticoagulation, systemic embolism associated with cardioversion is reduced to 0-1.1%.1,8 As a result, it is recommended that in patients with AF of longer than 48 hours, oral anticoagulant therapy with warfarin should be administered (goal for INR of 2.0 to 3.0) for a minimum of three weeks before cardioversion. In addition, because the return of atrial mechanical activity may be delayed for several weeks after the restoration of sinus rhythm, it is recommended that anticoagulation be continued for a minimum of four weeks after cardioversion. This will also decrease the chances of embolism if AF recurs.1
Transesophageal echocardiography (TEE) has been advocated by some as a screening tool to identify patients with AF of greater than 48 hours duration where there is no evidence of left atrial clot, allowing these patients to be cardioverted without prophylactic anticoagulation. However, studies have revealed that this technique is associated with a significant incidence of thromboembolic complications, resulting in current recommendations for anticoagulation in these patients.1,9,10 In addition, prior to cardioversion, even patients whose AF has lasted less than 48 hours should be anticoagulated with intravenous heparin, in order to cover the delays that may be encountered during medical treatment.1
Cardioversion Technique
Elective DC cardioversion has a higher rate of success in converting AF to sinus rhythm than does medical cardioversion,2,6 making it the most reliable means to restore sinus rhythm.1 The technique works by delivering an R-wave synchronized shock between two thoracic electrode paddles in an anesthetized patient. The paddles can be oriented anterolaterally or anteroposteriorly. A success rate of over 90% is achieved with either configuration.1 However, some data suggest that with regards to technical success, an anteroposterior defibrillator paddle position is superior to an anterolateral position and permits lower energy usage.11 Digoxin is usually stopped 24h before cardioversion, or at least a level is checked prior to cardioversion, since digitalis toxicity increases the risk of malignant ventricular arrhythmias.1 Generally, energy requirements depend on the duration of the AF (for instance, recent AF has coarser fibrillatory waves and requires lower energy). The first attempt at cardioversion is made with 200J. Increments of 100J are used if preceding shocks are not successful.1 Some studies have suggested that phamacologic agents, such as ibutilide, facilitate successful cardioversion of AF in patients who failed conventional external cardioversion. The medication is administered and cardioversion is attempted again.12 Internal cardioversion (beyond the scope of this article) is an option if external cardioversion has not been successful, and some studies indicate that this technique may work for AF of duration greater than one year but less than three years.13
Complications
A variety of short-lived arrhythmias can follow cardioversion, including premature atrial and ventricular beats, sinus pauses and junctional escape rhythms. These usually do not require treatment. There is a small risk of ventricular fibrillation, especially if there is poor synchronization of the DC shock and the native QRS complex. Pulmonary edema can also be a rare complication in the context of severely depressed left ventricular function.14 Thrombo-embolic complications are discussed above.1
Clinical Decisions
The decision to cardiovert a patient from atrial fibrillation to sinus rhythm is a clinical one. The most symptomatic patients will gain the most relief.1 Patients who have had atrial fibrillation for a shorter period will likely have less left atrial dilatation; therefore they have a greater probability of being maintained in sinus rhythm.
A reasonable approach is to attempt to cardiovert patients with AF of recent onset. Patients with chronic AF have two broad therapeutic options: 1) rate control and anticoagulation; and 2) cardioversion and maintenance of sinus rhythm. The comparative value of these approaches is still under investigation,8 although it might be reasonable to attempt to convert symptomatic patients to sinus rhythm. Conversion to sinus rhythm improves a patient's hemodynamic status and, as a result, his or her exercise tolerance. The left ventricular stroke volume and ejection fraction increase immediately after cardioversion, while the cardiac contractility remains unchanged. This implies that the improvement in hemodynamics is secondary to enhanced left ventricular diastolic filling, due to an increased cycle length and to the return of left atrial mechanical function (atrial "kick").15 This could be very important for patients with reduced left ventricular function.7 Other studies have demonstrated that changes in atrial electrophysiology and the atrial dilatation associated with chronic AF are reversible after cardioversion.16,17
Maintenance of Sinus Rhythm
While electrical cardioversion of AF to normal sinus rhythm is successful in more than 80% of cases, without antiarrythmic therapy, only 25% of patients will remain in sinus rhythm after one year.18 A cost-benefit analysis in the Annals of Internal Medicine suggests that cardioversion alone should be the initial management strategy for persistent, non-valvular atrial fibrillation. For a relapse of the arrhythmia, repeated cardioversion plus low-dose amiodarone was found to be cost-effective for patients at moderate to high risk for ischemic stroke.19 Another study has demonstrated that amiodarone is more effective than sotalol and propafenone for the prevention of recurrences of atrial fibrillation20 (see article).
Conclusions
Atrial fibrillation is a common, significant arrhythmia in the elderly. DC cardioversion is used in unstable cases, and as an elective procedure in cases of recent onset, to convert the patient to normal sinus rhythm. To avoid thromboembolic complications, patients with AF of greater than 48 hours duration should be anticoagulated with warfarin for three weeks prior to, and four weeks after cardioversion. Repeat cardioversion can be used for recurrent atrial fibrillation, and antiarrhythmic therapy, such as amiodarone, can be initiated to help maintain sinus rhythm. Cardioversion can also be used in symptomatic cases of AF of long duration, as duration has been shown to be less important than underlying heart disease in the success of conversion and maintenance of sinus rhythm.
References
- Beamish RE. (Ed.) Canadian Cardiovascular Society Consensus Conference on Atrial Fibrillation. Can J Cardiol 1996;12A:1A-61A.
- Aronow WS. Management of atrial fibrillation, ventricular arrhythmias and pacemakers in older persons: Management of the older person with atrial fibrillation. JAGS 1999;47(6): 740-8.
- Aronow WS, Ahn C, Gutstein H. Prevalence of atrial fibrillation and association of atrial fibrillation with prior and new thromboembolic stroke in older patients. J Am Geriatr Soc 1996;44:521-3.
- English KM, Channer KS. Managing atrial fibrillation in elderly people: Active management of atrial fibrillation should include elderly people. BMJ 1999;318:1088-9.
- Hampton JR. The management of atrial fibrillation in elderly patients. Age and Ageing 1999;28:249-50.
- Morris JJ Jr, Peter RH, McIntosh HD. Electrical conversion of atrial fibrillation: Immediate and long-term results and selection of patients. Ann Intern Med 1966;65:216-31.
- Nakazawa H, et al. Is there a place for late cardioversion of atrial fibrillation? Eur Heart J 2000;21:327-33.
- Lampert R, Ezekowitz MD. Management of arrhythmias. Clin in Ger Med 2000;16(3):593-618.
- Warren J, et al. Cardioversion from atrial fibrillation without prolonged anticoagulation with use of transesophageal echocardiography to exclude the presence of atrial thrombi NEJM 1993;328(11):750-5.
- Black IW, et al. Exclusion of atrial thrombus by transesophageal echocardiography does not preclude embolism after cardioversion of atrial fibrillation. A multicentre study. Circ 1994;89:2509-13.
- Botto GL, Politi A, Bonini W, Broffoni, T, Bonatti R. External cardioversion of atrial fibrillation: role of paddle position on technical efficacy and energy requirements. Heart 1999;82:726-30.
- Li H, et al. Usefulness of ibutilide in facilitating successful external cardioversion of refractory atrial fibrillation. Am J Cardiol. 1999;84:1096-8.
- Tse HF, et al. Long-term outcome in patients with chronic atrial fibrillation after successful internal cardioversion. Am J Cardiol. 1999;83:607-9.
- Levy S, et al. Atrial fibrillation: current knowledge and recommendations for management. Eur Heart J. 1998;19:1294-1320.
- Raymond RJ, et al. Cardiac performance early after cardioversion from atrial fibrillation. Am Heart J 1998;136(3):435-42.
- Hobbs WJC, et al. Reversal of atrial electrical remodeling after cardioversion of persistent atrial fibrillation in humans. Circ 2000:101;1145-51.
- Mattioli AV, et al. Serial evaluation of left atrial dimension after cardioversion for atrial fibrillation and relation to atrial function. Am J Cardiol. 2000;85:832-36.
- Coumel P, Thomas O, Leenhardt A. Drug therapy for prevention of atrial fibrillation. Am J Cardiol 1996;77(3):3A-9A.
- Catherwood E, et al. Cost-effectiveness of cardioversion and antiarrhythmic therapy in non-valvular atrial fibrillation. 1999;130(8):625-36.
- Roy D, et al. Amiodarone to prevent recurrence of atrial fibrillation. N Engl J Med 2000;342:913-20.