Click here to view the entire report from the 4th Canadian Colloquium on Dementia
Antiamyloid Immunotherapy: What Do the Blood Vessels Think?
Speaker: Steven M. Greenberg, MD, PhD, Director, Hemorrhagic Stroke Research Program, Massachusetts General Hospital; Associate Professor of Neurology, Harvard Medical School, Boston, MA, USA.
The final speaker, Dr. Steven Greenberg, focused on vascular issues related to antiamyloid immunotherapies.
Is Antiamyloid Therapy the Solution?
He began by addressing whether antiamyloid therapy might be considered a solution or might only contribute further to the problem of cognitive deterioration, as well as vascular pathology. As Dr. Relkin had discussed, earlier significant trials of the therapy resulted in a serious inflammatory response. What possible approaches might avoid this toxicity?
Historically, Dr. Greenberg observed, people taking anti-inflammatories to retard inflammation were thought to be at decreased risk for Alzheimer’s disease (AD), as inflammation was perceived as part of the cascade leading to AD-associated deterioration. That was turned on its ear in Dr. Dale Schenk’s work, as discussed by Dr. Relkin. Schenk’s work on immunization to beta-amyloid with mouse models appeared to generalize to humans. Indeed, individuals who have participated in immunization trials and who came to autopsy showed striking clearing of beta-amyloid deposition as a result of the therapy. The serious drawback of this treatment was the risk of meningoencephalitis, which led to initial discontinuation of the study.
The Toxicity of Antiamyloid Therapy
The adverse events included subacute cognitive decline, seizures, and white matter changes. These effects were reversible in some cases; however, two deaths were reported. The value of passive immunotherapy is still emerging-studies in which patients are receiving premade antiamyloid antibody therapies are ongoing, and some changes, reminiscent of the previous white matter changes on MRI, have been reported. Passive immunotherapies may not totally avoid toxicity and severe side effects.
Cerebral Amyloid Angiopathy and Inflammation
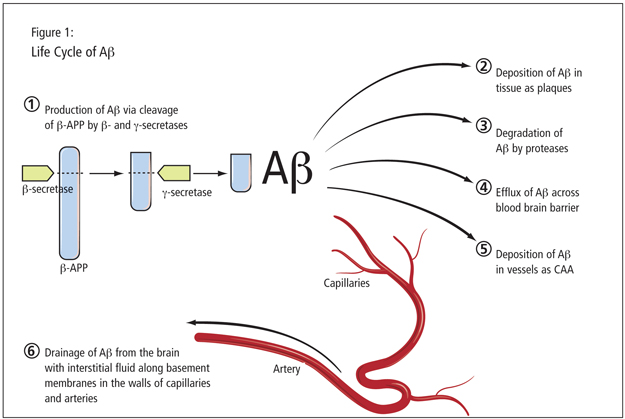
Dr. Greenberg stressed the connection between meningoencephalitis and cerebrovascular amyloid or cerebral amyloid angiopathy (CAA). This topic partners with the study of AD and amyloid. It involves essentially the same peptide, with a difference in Ab40 and Ab42 ratios. Dr. Greenberg showed an illustration of the life cycle of the amyloid peptide, a complex entity (Figure 1). Once formed, it might degrade, be deposited in the vessels, or pass out of the nervous system. There is a strong pathological overlap between Alzheimer’s and CAA. Research has uncovered the prevalence of vascular amyloid in almost every AD case. Dr. Greenberg also emphasized the presence of microbleeds which are, radiographically, a hallmark pathological feature found in a substantial set of AD patients. Such hemorrhages are also a cardinal feature of amyloid angiopathy, which tends to be worse in the parietal lobes where the hemorrhages are located. There is a nearly superimposable distribution of the hemorrhages when one examines individuals with AD; the microbleeds in AD patients occur in the same distribution as amyloid angiopathy. Bleeding in AD patients, Dr. Greenberg contended, suggests underlying amyloid angiopathy. There are virtually no AD patients without this associated vascular pathology.
Dr. Greenberg further considered what this vascular pathology present in AD patients has to do with the toxic response to beta-amyloid immunotherapy. The evidence comes from the pathology of vaccine-associated meningoencephalitis; the resemblance of iatrogenic toxicity to spontaneously occurring syndromes of amyloid angiopathy-related inflammation; and animal studies.
Dr. Greenberg showed brain pathology images from the two fatalities that occurred in the active vaccine study. Both autopsies revealed substantial clearing of amyloid plaques. However, the vascular amyloid remained present at an advanced stage, with evidence of vascular breakdown. There was significant inflammation around the vessels and an outpouring of inflammatory cells, mostly lymphocytes. Pathologically, it looked like there had been an inflammatory response to the vessels themselves. These results, he stated, are striking in light of spontaneously occurring syndromes of CAA-related inflammation: a sizeable subset of patients present with subacute cognitive changes or seizures. The pathology they exhibit is reminiscent of the changes seen in the active vaccine study-including advanced amyloid angiopathy and perivascular inflammation. There are focal cortical infarctions, possibly representing effects of inflammation. Their MRIs show subcortical changes in the white matter, extending to overlying grey matter. Radiographic as well as clinical data suggest similarities between the two processes. Dr. Greenberg compared the amount of white matter disease in study participants with spontaneously occurring CAA syndrome with patients with the iatrogenic pathology from the active vaccine study. Patients treated with anti-inflammatories improved clinically and showed corresponding improvement of white matter findings on MRI. There was a relapsing subgroup with bouts of spontaneously occurring CAA-people who came in with a second or third bout of the same syndrome-who showed repeated instances of return of the hyperintensities.
Studies of transgenic mice with robust plaque and vascular amyloid showed occurrences of hemorrhages in passive immunization. These findings with animal models represent another adverse occurrence and raise the question of what happens to vessels when amyloid is pulled from them.
So, Dr. Greenberg queried, if we suppose that vascular amyloid is the problem and is the trigger of adverse events in immune-based therapy, how is it avoided? Approaches to avoiding CAA-related toxicity might include better noninvasive detection of CAA; better identification of those at risk for CAA-related inflammation; and adjusting the mode of treatment.
Dr. Greenberg considered PET imaging for the noninvasive detection of amyloid angiopathy. He stated that Pittsburgh compound B (PIB) accumulates in regions with high levels of plaques. Because patients with amyloid angiopathy showed increased accumulation of this compound, he questioned whether this signifies that PIB binds to vascular amyloid. Dr. Greenberg argued that PIB predominantly accumulates in the occipital cortex, a region the most highly enriched in vascular amyloid. One may use a ratio of occipital to global PIB binding to determine how much amyloid would be present.
Talking about risk factors, Dr. Greenberg noted the overrepresentation of the APOE 4/4 genotype among patients with inflammatory CAA. This marker may represent susceptibility for CAA-related inflammation.
Finally, altered methods of treatment may minimize the risk of bad effects from vascular amyloid. Dr. Greenberg’s team has worked with multiphoton microscopy to look at the progression of vascular amyloid in living mice and mapped a predictable week-by-week progression of amyloid angiopathy in transgenic mice. The results were measured in a quantitative way across multiple imaging sessions. They wondered what would happen were they to directly apply beta amyloid antibodies to the surface of the brain in these transgenic mice. Results showed a decline in vascular amyloid. The direct application of antibodies to the surface of the brain reversed accumulation to the same extent vascular amyloid would tend to accumulate, with lack of toxicity. He stated that this suggests that beta-amyloid clearance can be done in a way that is protective of the vessels.
Conclusion
Dr. Greenberg concluded that the anti–beta-amyloid inflammatory response may be both beneficial when it is clearing amyloid, but harmful if it is causing tissue damage around it. There are clinical, radiological, animal, and other data suggesting that vascular amyloid contributes to vaccine-related toxicity. The possible approaches may involve altering the therapeutic approach, and determining who should be treated. Selecting subjects at low risk for adverse events may make antiamyloid immunotherapy a safer treatment.