Click here to view the entire report from the 4th Canadian Colloquium on Dementia
Alzheimer’s Disease: Treatments on the Horizon
Speaker: Dr. Serge Gauthier, MD, FRCPC, Director, Alzheimer Disease and Related Disorders Research Unit, McGill Centre for Studies in Aging, Montreal, QC.
Dr. Serge Gauthier focused his overview of current and future dementia treatments on the continuum of cognitive decline. He started with a “natural history of cognitive decline,” tracing cognitive deterioration’s trajectory from the earliest stages through profound impairment and death. The resulting picture is of a spectrum of cognitive abilities embracing the full range of health and pathology.
With this continuum in mind, Dr. Gauthier termed normal cognitive abilities as “pre-Mild Cognitive Impairment.” Such individuals might have subjective memory complaints, but these are undemonstrated in testing.
The next position on the spectrum was “Amnestic MCI” or “Predementia Alzheimer’s Disease.” This may be a prodromal phenomenon, Dr. Gauthier explained. These individuals he called “the forgetfuls,” noting that some but not all have pre-dementia Alzheimer’s disease (AD). Current symptomatic treatments for this stage include antidepressants and cholinesterase inhibitors (ChEI).
The choice of medication and the development of future treatments is connected to the concept of disease progression (Figure 1). He advised the audience to recall the difference between disease-modifying versus symptomatic drugs. With the latter, no improvement in the patient’s condition is expected, the criterion being only stabilization.
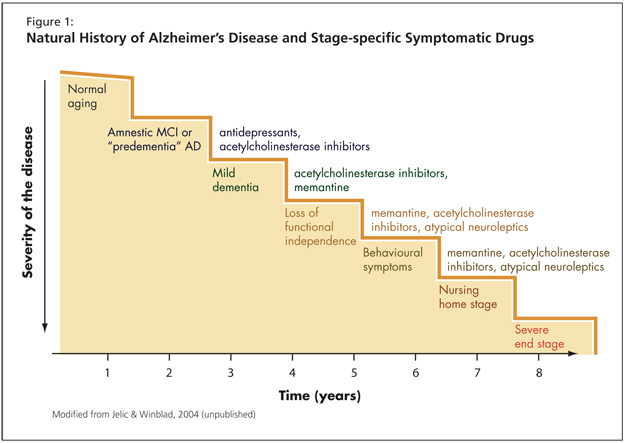
Dr. Gauthier cited a study by Winblad et al. from Neurology (2001) as evidence of donepezil’s benefit.1 He emphasized that this is a class effect: clinicians can expect improvement over baseline for 9-12 months for mild to moderate levels of impairment. Individuals with moderate to severe impairment living at home can expect improvement for about 6 months. In terms of cognition, patients will improve for anywhere from 6-12 months, depending on the severity of their cognitive decline.
Dr. Gauthier addressed the issue of combining agents, namely, memantine and donepezil in patients with mild dementia. He noted that studies have found additional improvement in cognition scores with the combined treatment over a period of 6 months.
In terms of agents on the horizon, Dr. Gauthier discussed the coming rivastigmine transdermal patch. The theoretical benefits, he stated, are clear. Recent studies confirm demonstrated benefit over baseline in MMSE scores. Similar benefits were demonstrated on the ADCS-ADL. The benefit with the patch is that higher doses may be achievable, without compromise to safety and tolerability.
Dr. Gauthier emphasized the value of Goal Attainment Scaling, as discussed by Dr. Kenneth Rockwood in the Canadian Medical Association Journal. Individual goal setting, in combination with galantamine, can be of value to patient care when real improvement in disease expression and progression is not expected, he noted.
The key idea, Dr. Gauthier stressed, is that symptomatic treatments for mild to severe stages of AD and related dementias are already available.
Modifying Disease among Mild AD Patients
Dr. Gauthier discussed disease modification among the patient group on the mild end of the spectrum of AD expression. In presenting details of two studies, he mentioned the caveat that the traditional study duration is sometimes inadequate to chart improvement among this treatment group. Eighteen months may be insufficient with mild AD patients.
Tramiprosate & Taranflurbil
Dr. Gauthier discussed treatment trials of the agent tramiprosate (Alzhemed®), an agent that binds to the soluble amyloid Ab. Phase III data from the clinical trial did not demonstrate statistical significance in favour of tramiprosate, with respect to the primary endpoints over 18 months of treatment. An advisory board of experts is currently reviewing data to see if benefit could have been measured differently. A European Phase III clinical trial is ongoing and could be modified based on North American findings.
The second agent Dr. Gauthier considered was taranflurbil (Flurizan®), a modulator of gamma secretase, currently in Phase II testing under lead investigator Dr. Sandra Black. The Phase II effort is to determine the most appropriate dosage. Patients in the study’s Canadian arm continued on the regime for an extra year. Key results showed that patients with mild AD who received a high dose of the medication obtained MMSE results indicating stabilization. Another surprise from the Phase II data was the statistically significant reduction of the psychiatric side effects. The protocol is ongoing for Phase III, and results are expected in December 2008.
Dr. Gauthier observed that there is room for improvement in the responder analysis: he offered that the 18-month baseline is not as useful a measure as it could be, and that a responder analysis would be useful. Further, delay to events-that is, the delayed emergence of significant psychiatric symptoms-would be a more meaningful measure of benefit, according to Dr. Gauthier. Disease-modifying drugs are under testing in mild AD, the primary hypothesis being that amyloid deposition is a major cause of the disease. The question remains, is this stage of dementia the best one for such pharmacotherapies?
A Closer Look at Mild Cognitive Impairment
Mild cognitive impairment (MCI), Dr. Gauthier emphasized, is neither a diagnosis nor a disease, but a way of defining cognitive deficit that has clinical utility. There will never be a drug for MCI, he stated. He presented results of a joint Canada-U.S. study comparing Vitamin E, donepezil, and placebo.2 The study found no difference in conversion rates at 3 years for any of the groups. At present, there is nothing to be given for MCI.
However, the data are different for predementia Alzheimer’s. Dr. Gauthier mentioned his recent position paper considering research criteria for the diagnosis of AD, published in 2007 in Lancet Neurology and advocating a change in the core diagnostic criteria.3
The ultimate effect of the changed criteria is to extend the determination of Alzheimer’s in a predementia stage. A diagnosis of the predementia stage of AD would become a clinical possibility and would be considered amenable to experimental treatments, with progression to dementia as a primary endpoint.
Conclusion: Controllable Risk Factors for Cognitive Decline
Dr. Gauthier predicted that control of risk factors will be integrated as part of the general stage-specific intervention schema. He noted specifically that education, dietary, and lifestyle factors have been correlated with cognitive effects. Incorporating measures to improve these factors will be integral to efforts to slow the conversion rate of pre-AD memory to dementia, and strategies to target them (such as interventions to control vascular risk factors) will be of key interest to researchers in coming years. This is fuelling study of agents such as ginkgo biloba. Dr. Gauthier advised predicted growing interest in the “triple approach” of diet (particularly the Mediterranean diet, shown to reduce risk by ~40%), exercise, and cognitive training. Overall, the focus of research and study for the next 5 years will likely reflect a preventive approach to cognitive impairment and the dementing syndromes.
References
- Winblad B, Engedahl K, Soininen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 2001;57:489-95.
- Peterson RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005;352:2379-88.
- Dubois M, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007;6:734-46.